Description
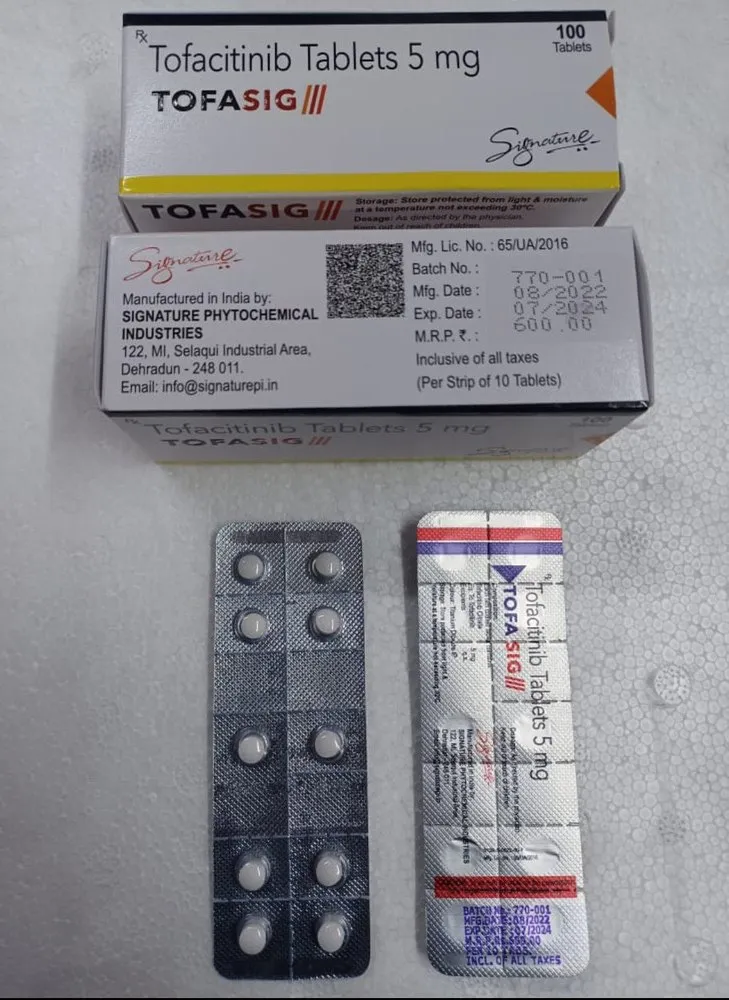
TOFASIG TABLET contains Tofacitinib, an advanced Janus kinase (JAK) inhibitor, used in the treatment of moderate to severe autoimmune diseases. By inhibiting the JAK-STAT signaling pathway, TOFASIG helps regulate the immune system and reduce symptoms such as joint pain, swelling, and stiffness.
It is commonly prescribed for patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ulcerative colitis (UC) who have had an inadequate response to other conventional therapies like methotrexate or TNF inhibitors.
Janus Kinase (JAK) Inhibitor – Disease-Modifying Anti-Rheumatic Drug (DMARD)
Tofacitinib selectively inhibits JAK enzymes (primarily JAK1 and JAK3), which play a crucial role in cytokine signaling within the immune system. By blocking these enzymes, TOFASIG reduces inflammation and halts immune-mediated damage to joints and tissues.
-
Rheumatoid Arthritis (RA)
-
Psoriatic Arthritis (PsA)
-
Ulcerative Colitis (UC)
-
Other inflammatory autoimmune diseases (as per physician guidance)
-
Reduces joint inflammation, stiffness, and pain
-
Prevents progression of autoimmune damage
-
Improves physical function and quality of life
-
Oral administration offers convenience over injectables
-
Can be used in patients intolerant to biologics
-
Commonly prescribed as 5 mg twice daily or extended-release formulations once daily, depending on the indication
-
Must be taken exactly as directed by the physician
-
Monitoring required for blood counts, liver enzymes, and lipid levels
-
Not recommended during pregnancy or breastfeeding unless prescribed
-
Regular screening for infections (e.g., TB, hepatitis) is required before starting
-
Use with caution in patients with liver or kidney disease
-
Can increase risk of serious infections, blood clots, or malignancy with long-term use
-
Upper respiratory infections
-
Headache, diarrhea
-
Increased liver enzymes
-
High cholesterol levels
-
Risk of blood clots and serious infections in rare cases
BLUEPILL EXPRESS is a globally trusted third-party manufacturer and exporter of high-standard medicines. TOFASIG TABLET is developed under rigorous GMP guidelines and is tested for consistency, potency, and safety.
We offer:
-
WHO-GMP & ISO-certified production
-
Custom branding & private labeling services
-
Regulatory documentation for global markets
-
Bulk export to over 50 countries
TOFASIG TABLET is part of our expanding global portfolio, exported to healthcare providers and distributors in:
-
Latin America
-
Southeast Asia
-
Africa & the Middle East
-
CIS Countries
-
Europe & Oceania
We ensure timely delivery, proper documentation, and high-quality compliance in all international transactions.